Abstract
Background: We recently demonstrated that diffuse myocardial fibrosis is a common and novel mechanism of heart disease in sickle cell anemia (SCA) that is strongly associated with diastolic dysfunction (Niss et al. Blood 2017). Diffuse myocardial fibrosis can be detected noninvasively by cardiac magnetic resonance imaging (CMR).The temporal evolution of diffuse myocardial fibrosis in SCA has not been studied before.
Methods: This is an ongoing, prospective, longitudinal study to characterize the cardiomyopathy of SCA (NCT02410811). We used CMR measurements of native T1 and extracellular volume fraction (ECV) to detect and quantify diffuse myocardial fibrosis. ECV was calculated from pre- and post-gadolinium T1 measurements of blood and myocardium. Focal myocardial fibrosis was detected by late gadolinium enhancement (LGE). Diastolic function was assessed by echocardiography. We compared paired CMR studies from study entry and 1-year follow-up.
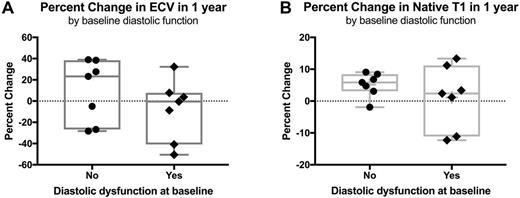
Results: We studied 25 individuals with SCA who were 23±11 years of age (mean±SD). All had markedly increased ECV at baseline compared to controls (0.44±0.08 vs. 0.26±0.02, P<0.0001), indicating the presence of diffuse myocardial fibrosis; 1 also had LGE-defined focal myocardial fibrosis. At baseline, 8 (32%) had normal diastolic function, 17 (71%) had diastolic abnormalities, and 7 (29%) met the definition for diastolic dysfunction. The second (1-year follow-up) CMR was performed 12±0.4 months after the baseline. For all participants, the mean difference (year 1 - baseline) in ECV was -0.03 (95% CL: -0.08, 0.03; P=0.34), indicating general stability of the phenotype over 1 year. Similarly, the mean difference in native T1 values was 19 ms (95% CL: -10, 48; P=0.19). None developed new focal fibrosis. Participants with normal systolic and diastolic function at baseline (N=7) had median increases of 23% and 6% in ECV (Figure, Panel A, •) and native T1 (Figure, Panel B, •), respectively, at 1-year follow-up, compared to those with diastolic dysfunction at baseline (N=7) who had a median -0.46% decrease in ECV (Figure, Panel A, ♦; P=0.32) and 2% increase in native T1 (Figure, Panel B, ♦; P=0.45).
Conclusions: Diffuse myocardial fibrosis is a common feature of SCA. Overall, the CMR measurements of diffuse fibrosis, native T1 and ECV, are stable over 1 year in SCA, demonstrating their utility to monitor anti-fibrotic or disease-modifying therapy for the cardiomyopathy of SCA. Individuals with normal heart function at baseline appear to be at risk of progressive myocardial fibrosis.
Quinn: Global Blood Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal